The latest report published by IMARC Group, titled “Ventricular Assist Devices Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2023-2028”, offers a comprehensive analysis of the industry, which comprises insights on ventricular assist devices market growth. The report also includes competitor and regional analysis, and contemporary advancements in the global market.
The global ventricular assist devices market size reached US$ 1.7 Billion in 2022. Looking forward, IMARC Group expects the market to reach US$ 3.1 Billion by 2028, exhibiting a growth rate (CAGR) of 9.7% during 2023-2028.
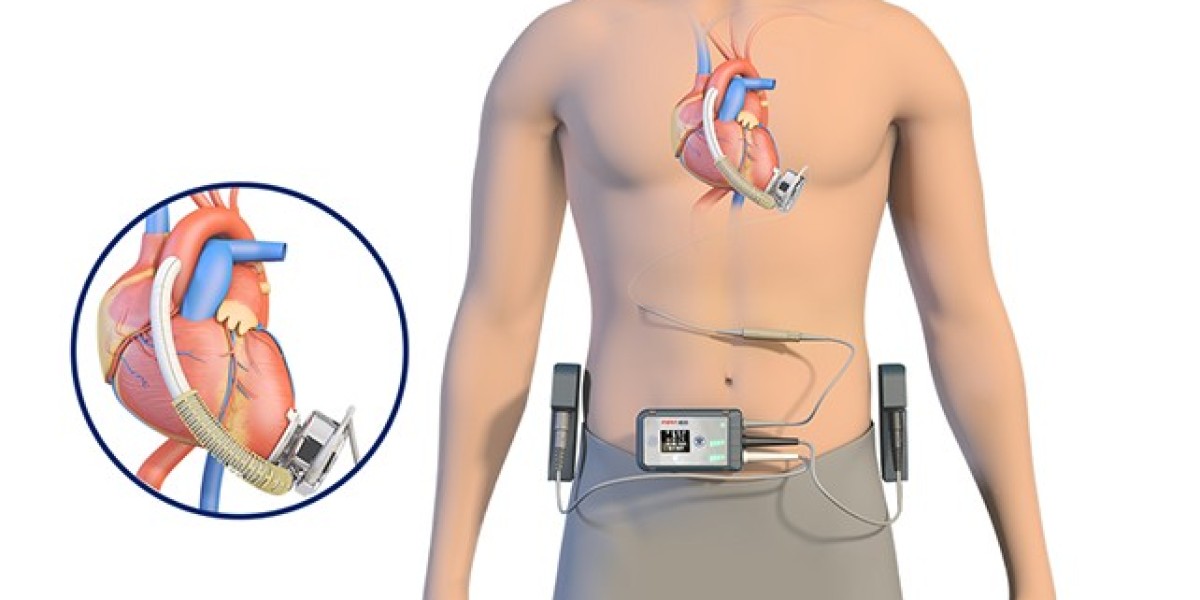
Ventricular assist devices (VADs) represent a pivotal advancement in the field of medical technology, specifically designed to provide mechanical support to a weakened or failing heart. These life-saving devices come in various types, including left ventricular assist devices (LVADs) and right ventricular assist devices (RVADs), each serving a unique purpose in augmenting cardiac function. They are implanted within the body and work by assisting the heart in pumping blood, either on a short-term basis while awaiting a heart transplant or as a long-term solution for individuals ineligible for transplantation. The advantages of VADs are profound. They provide a bridge to transplantation, extending the lives of patients awaiting heart transplants and improving their quality of life. Additionally, they offer destination therapy for those ineligible for transplantation, enhancing survival rates and promoting cardiac health.
Request to Get the Sample Report: https://www.imarcgroup.com/ventricular-assist-devices-market/requestsample
Market Trends:
The global market is majorly driven by the changing healthcare landscape. VADs are emerging as a critical approach to address this important healthcare concern as the prevalence of heart failure keeps increasing internationally. The aging population, coupled with an increase in lifestyle-related factors contributing to heart disease, is driving the demand for VADs. Along with this, technological advancements in device design and miniaturization have made VADs more accessible and safer for patients. Additionally, the shortage of donor hearts for transplantation is providing a boost to the utilization of VADs as a bridge to transplantation. In addition, collaborations between medical device manufacturers, research institutions, and healthcare providers are fueling innovation in this sector, resulting in more efficient and patient-friendly VADs. Furthermore, regulatory support and reimbursement policies are also facilitating market growth, making these devices accessible to a broader patient population.
View Full Report with TOC & List of Figure: https://www.imarcgroup.com/ventricular-assist-devices-market
Competitive Landscape:
The competitive landscape of the market has been studied in the report with the detailed profiles of the key players operating in the market.
- Abbott Laboratories
- Abiomed Inc.
- Berlin Heart GmbH (Syscore GmbH)
- Bivacor Inc.
- Calon Cardio
- Cardiacassist Inc. (LivaNova PLC)
- CHF Solutions Inc.
- Jarvik Heart Inc.
- MAQUET GmbH (Getinge)
- Medtronic Inc.
- Syncardia Systems LLC (Versa Capital Management LLC)
- TandemLife (LivaNova PLC)
- Terumo Corporation
Ventricular Assist Devices Market Segmentation:
Our report has categorized the market based on region, product, flow type, product type, application, and end user.
Breakup by Product:
- Left Ventricular Assist Device (LVAD)
- Right Ventricular Assist Device (RVAD)
- Biventricular Assist Device (BiVAD)
- Others
Breakup by Flow Type:
- Pulsatile Flow
- Non-Pulsatile or Continuous Flow
Breakup by Product Type:
- Implantable Ventricular Assist Devices
- Non-implantable Ventricular Assist Devices
Breakup by Application:
- Bridge-to-Transplant (BTT) Therapy
- Destination Therapy
- Bridge to Recovery and Bridge to Candidacy
Breakup by End User:
- Ambulatory Surgery Centers
- Hospital
- Others
Breakup by Region:
- North America (United States, Canada)
- Europe (Germany, France, United Kingdom, Italy, Spain, Others)
- Asia Pacific (China, Japan, India, Australia, Indonesia, Korea, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa (United Arab Emirates, Saudi Arabia, Qatar, Iraq, Other)
Key highlights of the report:
- Market Performance (2017-2022)
- Market Outlook (2023-2028)
- Porter’s Five Forces Analysis
- Market Drivers and Success Factors
- SWOT Analysis
- Value Chain
- Comprehensive Mapping of the Competitive Landscape
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Contact US:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145 | United Kingdom: +44-753-713-2163