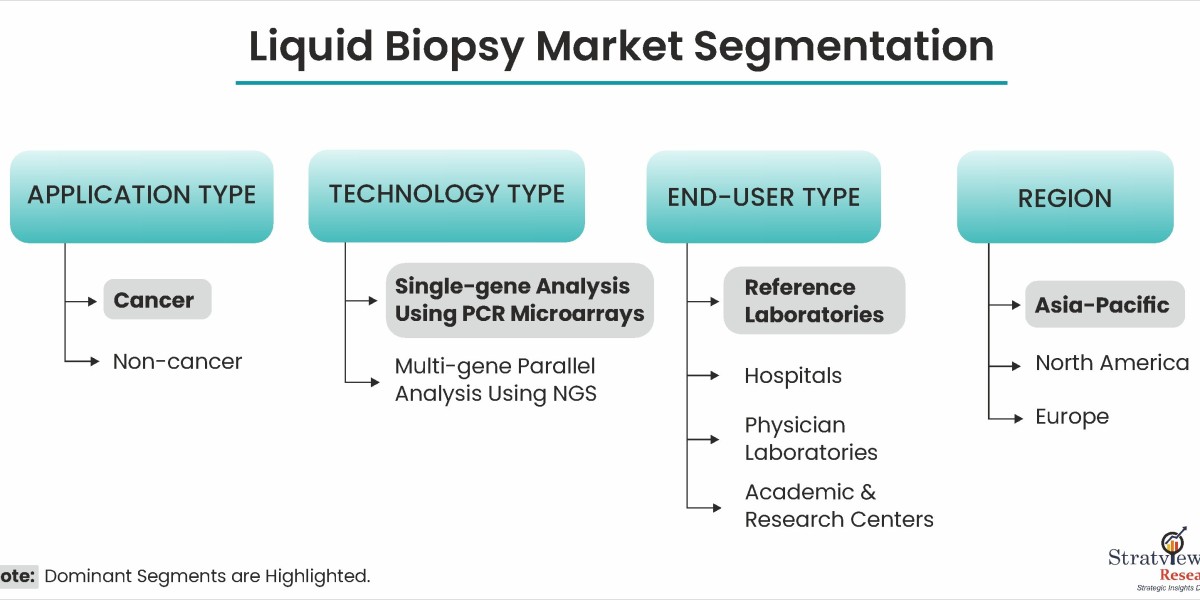
According to Stratview Research, the liquid biopsy market was estimated at USD 2.83 billion in 2022 and is likely to grow at a CAGR of 17.99% during 2023-2028 to reach USD 7.64 billion in 2028.
In the fight against cancer, early detection is often the key to successful treatment and improved patient outcomes. Traditional diagnostic methods, such as tissue biopsies, have long been the gold standard for diagnosing cancer. However, these procedures can be invasive, costly, and sometimes inconclusive. Enter liquid biopsy – a non-invasive alternative that is revolutionizing cancer diagnosis by providing clinicians with valuable insights into a patient's cancer status through the analysis of biomarkers in bodily fluids. As the liquid biopsy market continues to evolve, it is reshaping the landscape of cancer diagnosis, offering new hope to patients and clinicians alike.
The Promise of Liquid Biopsy in Cancer Diagnosis
Liquid biopsy offers several advantages over traditional tissue biopsies in the diagnosis and monitoring of cancer. By analyzing circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and other biomarkers present in blood, urine, or other bodily fluids, liquid biopsy can provide real-time information about a patient's cancer status with minimal risk and discomfort. This non-invasive approach is particularly valuable for patients with tumors that are difficult to access or sample, such as those in the brain or pancreas.
Moreover, liquid biopsy has the potential to detect cancer at an earlier stage when treatment is most effective, as well as to monitor tumor progression and treatment response over time. This allows clinicians to personalize treatment strategies based on the unique molecular profile of each patient's cancer, leading to more targeted and effective therapies.
Expanding Applications in Oncology
The applications of liquid biopsy in oncology are vast and continually expanding. In addition to its role in diagnosing cancer, liquid biopsy can be used to monitor minimal residual disease (MRD) after surgery or chemotherapy, helping to identify patients at high risk of relapse and guide treatment decisions. Liquid biopsy can also be used to detect the emergence of drug resistance mutations, allowing clinicians to adjust treatment regimens accordingly and prolong patient survival.
Furthermore, liquid biopsy holds promise for screening high-risk populations for cancer, such as individuals with a family history of cancer or those with genetic predispositions. By detecting cancer at an early stage, liquid biopsy has the potential to save lives and reduce the burden of the disease on patients, families, and healthcare systems.
Driving Forces Behind Market Growth
Several factors are driving the rapid expansion of the liquid biopsy market in oncology, including advancements in technology, increasing demand for personalized medicine, and growing awareness of the limitations of traditional diagnostic methods. Next-generation sequencing (NGS), digital PCR, and other molecular technologies have significantly enhanced the sensitivity and specificity of liquid biopsy assays, enabling the detection of rare mutations and biomarkers with unprecedented accuracy.
Moreover, the shift towards value-based healthcare and the emphasis on early detection and prevention are driving demand for non-invasive diagnostic solutions that can improve patient outcomes and reduce healthcare costs. Liquid biopsy offers a promising solution to these challenges, providing clinicians with the tools they need to make more informed treatment decisions and improve patient care.
Future Directions and Challenges
Despite its many promises, liquid biopsy still faces several challenges, including standardization of protocols, validation of biomarkers, and regulatory approval. Standardization of pre-analytical and analytical procedures is essential to ensure the reproducibility and reliability of liquid biopsy results across different laboratories and platforms. Additionally, further research is needed to validate the clinical utility of liquid biopsy biomarkers and establish their prognostic and predictive value in different cancer types and patient populations.
Looking ahead, the future of the liquid biopsy market in oncology looks promising, with continued advancements in technology, increasing adoption by healthcare providers, and expanding applications across the cancer care continuum. As we continue to revolutionize cancer diagnosis through the evolving landscape of liquid biopsy, we can expect to see further innovations that will transform the way we detect, treat, and ultimately, conquer cancer.