According to a new report published by UnivDatos Markets Insights, the Clinical Trials Management System Market was valued at USD 1 billion in 2021 is expected to grow at a CAGR of 14% from 2022-2030. The analysis has been segmented into Type (Enterprise-Based and Enterprise-Based); Delivery Mode (Web-Based CTMS, On-Premises, and Cloud-Based); Component (Software and Services); End-Users (Pharmaceutical Biopharmaceutical Companies, Clinical Research Organization, and Healthcare Providers); and Region/Country
Get the inside scoop with Sample report @ https://univdatos.com/report/clinical-trials-management-system-market/get-a-free-sample-form.php?product_id=38222
The clinical trials management system market report has been aggregated by collecting informative data on various dynamics such as market drivers, restraints, and opportunities. This innovative report makes use of several analyses to get a closer outlook on the clinical trials management system market. The clinical trials management system market report offers a detailed analysis of the latest industry developments and trending factors in the market that are influencing the market growth. Furthermore, this statistical market research repository examines and estimates the clinical trials management system market at the global and regional levels.
Key Market Dynamics
The increasing demand for more efficient and streamlined clinical trial processes driving the growth of the market. Because these clinical trials are complex and involve several stakeholders, including investigators, research coordinators, data managers, and regulatory bodies, all of them need to work together seamlessly to ensure the success of the trial. Clinical trial management systems (CTMS) help to facilitate this process by providing a centralized platform for managing all aspects of a clinical trial, from patient recruitment, enrolment to data collection, and analysis of the data. It also reduces the time and resources needed to conduct a clinical trial and improves the accuracy of the data collection. Moreover, with the increasing complexity of clinical trials, there is a growing demand for real-time data monitoring and analysis. A CTMS can help to provide this by allowing for the integration of various data sources and providing advanced analytics capabilities. As the demand for more efficient and effective clinical trials continues to grow, the CTMS market is expected to see significant growth during the forecast period.
FIG. 1 Global Clinical trials management system Market Revenue, by region 2021 (% Share) |
|
Source: UnivDatos Market Insights
Ask for Report Customization @ https://univdatos.com/report/clinical-trials-management-system-market/get-a-free-sample-form.php?product_id=38222
COVID-19 Impact
The Clinical Trials Management System (CTMS) market was significantly affected by the COVID-19 pandemic. This included studies being delayed, barriers in patient recruitment, and disruptions in clinical trials. However, several strategic steps taken by governments, regulatory bodies, and market stakeholders to ensure the continuity of RD reduced the negative impact gradually. Moreover, government guidelines for clinical trials during the pandemic also boosted the clinical trials which was helpful for the growth of the market during the pandemic. For instance, the U.S. FDA published a guidance document in March 2020, on the conduct of clinical trials of medical products during the pandemic.
The global clinical trials management system market report is studied thoroughly with several aspects that would help stakeholders in making their decisions more curated.
By Type, the market is bifurcated into enterprise-based and site-based. Among these, enterprise-based captured the majority share of the clinical trials management system market in 2021.
On the basis of component, the market is bifurcated into software and services. The software category held a major share of the clinical trials management system market in 2020 and is expected to grow at a substantial CAGR during the forecasted period.
Clinical trials management system Market Geographical Segmentation Includes:
North America (U.S., Canada, and the Rest of North America)
Europe (Germany, UK, France, Italy, Spain, Rest of Europe)
Asia-Pacific (China, India, Japan, and Rest of Asia-Pacific)
Rest of the World
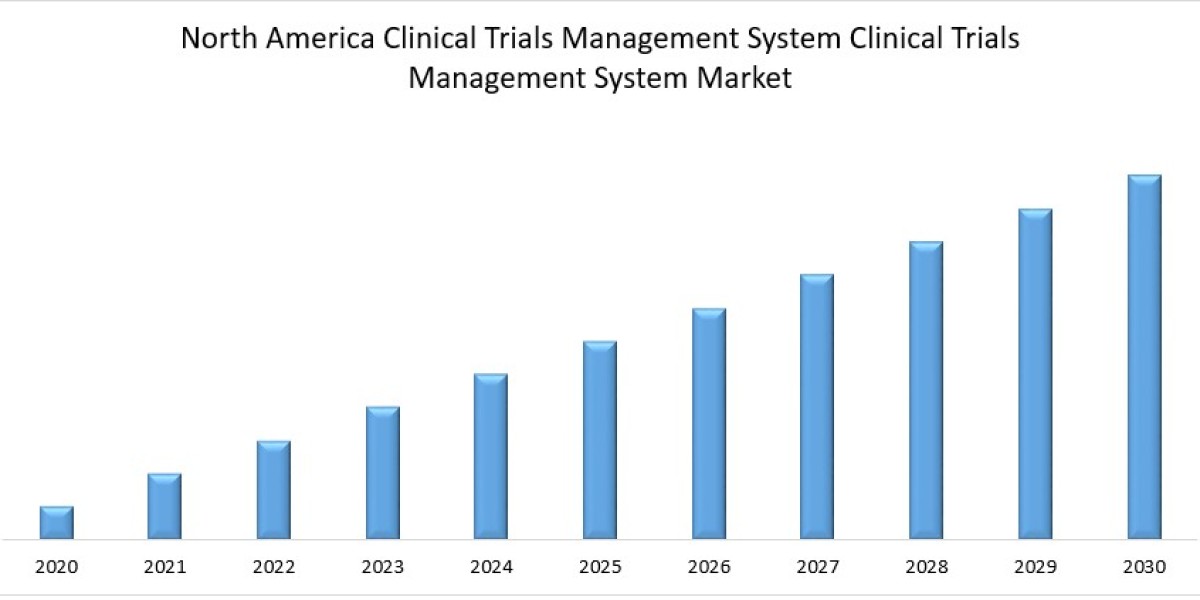
North America is anticipated to grow at a substantial CAGR during the forecast period owing to the rising adoption of technology, growing RD, and the availability of major market players in the region. Also, there are some other factors that drive the growth of the market such as favorable regulatory policies and increasing investment by pharmaceutical companies, the growing number of clinical trials, and government funding for research studies. For instance, according to the WHO International Clinical Trials Registry Platform, around 10,870 clinical trials were registered in the U.S. in 2021.
List of key start-ups in the North America Region:
Company Name | Company Logo | Year of Establishment |
Medable |
| 2022 |
Curebase Inc. |
| 2017 |
Hawthorne Effect |
| 2015 |
Suvoda LLC |
| 2012 |
Curavit Clinical Research |
| 2019 |
The major players targeting the Global clinical trials management system market:
Company Name | Company Logo | Revenue (2021) |
IQVIA Inc | USD XX Bn | |
Dassault Systmes | USD XX Bn | |
Oracle | USD XX Bn | |
Datatrak Int. | USD XX Bn | |
Clario | USD XX Bn | |
SimpleTrials | USD XX Bn | |
Calyx | USD XX Bn | |
RealTime Software Solutions, LLC | USD XX Bn | |
Laboratory Corporation of America Holdings | USD XX Bn | |
PHARMASEAL |
Comments
|