The In Vitro Diagnostics market in Europe is undergoing significant transformation, driven by rapid technological advancements, demographic shifts, regulatory changes, and evolving healthcare needs. These factors are reshaping the industry, offering new opportunities and challenges. This article provides insights into the current market landscape and offers predictions for the future of IVD in Europe.
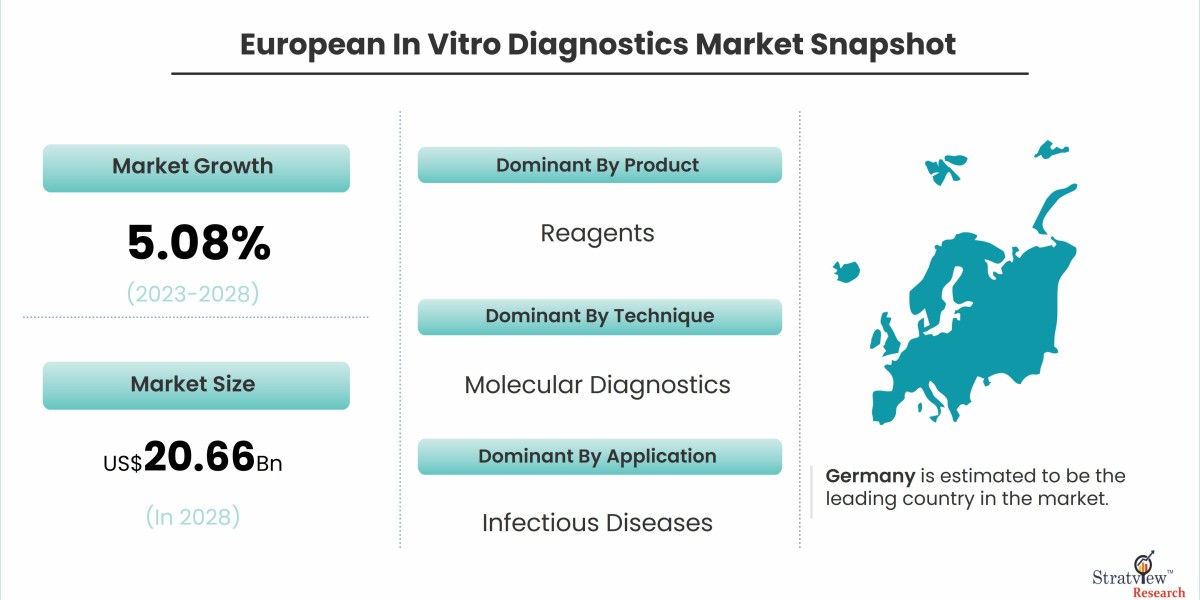
According to Stratview Research, the European in vitro diagnostics market was estimated at USD 15.29 billion in 2022 and is likely to grow at a CAGR of 5.08% during 2023-2028 to reach USD 20.66 billion in 2028.
Current Market Insights
Technological Innovations
Technological advancements are at the core of the IVD market's evolution. Innovations such as next-generation sequencing (NGS), digital pathology, and point-of-care testing (POCT) are revolutionizing diagnostics. NGS allows for comprehensive genetic analysis, aiding in the early detection and personalized treatment of diseases like cancer. Digital pathology enables remote diagnosis and collaboration, while POCT provides rapid results outside traditional laboratory settings, enhancing patient care and convenience.
Demographic Shifts
Europe’s aging population is significantly impacting the IVD market. The prevalence of chronic diseases such as diabetes, cardiovascular conditions, and cancer is rising as the population ages. Early detection and continuous monitoring of these diseases are crucial for effective management, leading to increased demand for diagnostic tests. This demographic trend is a major driver of market growth.
Regulatory Environment
The introduction of the In Vitro Diagnostic Regulation (IVDR) in Europe is reshaping the regulatory landscape. The IVDR aims to ensure the safety and efficacy of IVD products by imposing stricter requirements for testing and certification. While compliance with these regulations presents challenges, it also promotes high standards and fosters innovation. Companies that successfully navigate these changes can enhance their market position by offering reliable and compliant diagnostic solutions.
Integration of Artificial Intelligence and Big Data
Artificial intelligence (AI) and big data are transforming the IVD sector by enhancing diagnostic accuracy and operational efficiency. AI algorithms can analyze large datasets quickly, identifying patterns and predicting disease outcomes more effectively than traditional methods. This capability is particularly valuable in areas such as oncology and genomics, where precise diagnostics are critical. The integration of AI and big data is expected to drive further innovation and efficiency in the IVD market.
Future Predictions
Continued Technological Advancements
Technological innovation will continue to be a key driver of the IVD market. Advances in molecular diagnostics, digital health, and AI will further enhance the accuracy, speed, and accessibility of diagnostic tests. Emerging technologies such as liquid biopsy and CRISPR-based diagnostics hold significant promise for early disease detection and personalized medicine. As these technologies mature and become more integrated into clinical practice, they will drive market growth and improve patient outcomes.
Growth of Personalized Medicine
The shift towards personalized medicine will significantly impact the IVD market. Personalized medicine tailors treatment based on an individual’s genetic profile and specific disease characteristics, requiring precise and reliable diagnostic tools. The trend towards personalized medicine is expected to drive the development of specialized and companion diagnostics, which are crucial for the success of targeted therapies. This shift will lead to more individualized and effective patient care.
Expansion of Point-of-Care Testing
Point-of-care testing (POCT) will continue to grow in importance due to its ability to provide rapid results in various healthcare settings. Technological advancements in POCT devices will expand the range of tests available, making them more accurate and easier to use. The growing demand for immediate diagnostic information in emergency rooms, clinics, and home care settings will drive the expansion of POCT, making it a critical component of the IVD market.
Increasing Role of Digital Health
Digital health technologies, including telemedicine and remote diagnostics, will play an increasingly important role in the IVD market. The COVID-19 pandemic accelerated the adoption of these technologies, and this trend is expected to continue. Remote diagnostic tools, facilitated by digital health platforms, will improve access to healthcare services, particularly in remote and underserved areas. This shift will enhance patient convenience and support the growth of the IVD market.
Focus on Sustainability
Sustainability will become a key focus in the IVD market, driven by regulatory requirements and increasing awareness of environmental issues. Companies will need to adopt sustainable practices in the development, production, and disposal of IVD products. This focus on sustainability will drive innovation in materials, processes, and technologies, leading to more environmentally friendly diagnostic solutions.
Conclusion
The European In Vitro Diagnostics market is poised for significant growth, driven by technological advancements, demographic shifts, regulatory changes, and evolving healthcare needs. The integration of AI, big data, and digital health technologies will enhance diagnostic accuracy and operational efficiency, while the shift towards personalized medicine will drive the development of specialized diagnostics. The expansion of point-of-care testing and the increasing focus on sustainability will further shape the market landscape. As these trends continue to evolve, stakeholders in the IVD industry must remain agile and innovative to capitalize on the opportunities and navigate the challenges ahead. The future of the IVD market in Europe looks promising, with the potential for substantial advancements in diagnostic capabilities and improved patient outcomes.