Global real world evidence solutions market is expected to register a CAGR of approx. 13% over the period of 2022-2028. Real-world Evidence (RWE) is clinical evidence gathered through RWD analysis on the use and potential benefits or risks of a medical product. Randomized trials, large simple trials, pragmatic trials, and observational studies are just a few of the study types or analyses that can result in RWE (prospective or retrospective). In addition to reducing clinical trial costs and lengthening development timelines, RWE also increases the likelihood that technical and regulatory success will be achieved.
Access a Sample copy of the report browse through- https://univdatos.com/get-a-free-sample-form-php/?product_id=30787
The global real world evidence solutions market is increasing the need for additional information on compliance, epidemiology, and the demand for drug safety among investors, medical aid providers, and others thus, rising the adoption of inorganic growth strategies among key industry players. For instance, In April 2021, IQVIA Holdings Inc. announced the launch of the Connected Intelligence Platform. This platform is an innovative approach that enables customers in the life sciences industry to discover powerful new insights, drive smarter decision-making, and get treatments to patients faster. Owing to these glaring statistics industries are focused on launching innovative and technologically effective solutions thus increasing the demand for real world evidence solutions market. Moreover, many life sciences companies are adopting cloud technologies owing to the speed, flexibility, security, and scalability they provide is fueling the demand for the real world evidence solutions market. Furthermore, the rising market demand for EHR in which EHRs are the most frequent type of healthcare information that aids in executing outcome-based investigations is expected to drive the market for real world evidence solutions market in the coming years.
For a detailed analysis of the Global Real World Evidence Solutions Market browse through- https://univdatos.com/report/real-world-evidence-solutions-market/
Based on the component, the real world evidence solutions market is segmented into services and data sets. The services segment accounted for a significant market share in 2020, and it is estimated that it will grow rapidly during the projected timeframe due to the high adoption of real world services by pharmaceutical and biotechnology companies and healthcare providers. Because of the increased demand for comprehensive evidence services throughout the product lifecycle, real-world evidence solution vendors are likely to increase their investments throughout the drug development cycle.
Based on the application, the market is fragmented into pharmaceutical & medical device companies, healthcare payers, healthcare providers, and others. The pharmaceutical & medical device companies segment grabbed a considerable market share, and it is expected to grow at a significant CAGR during the forecast period due to the rising importance of RWE studies in the drug approval process, assessing drug performance in real world settings, and preventing drug recalls. RWE can help and advance a variety of processes, including determining which patient cohorts will benefit the most from an intervention, informing regulatory approvals, supplementing data from randomized controlled trials, assisting with reimbursement efforts, and expanding indications of use.
For a detailed analysis of the Global Real World Evidence Solutions Market browse through- https://univdatos.com/report/real-world-evidence-solutions-market/
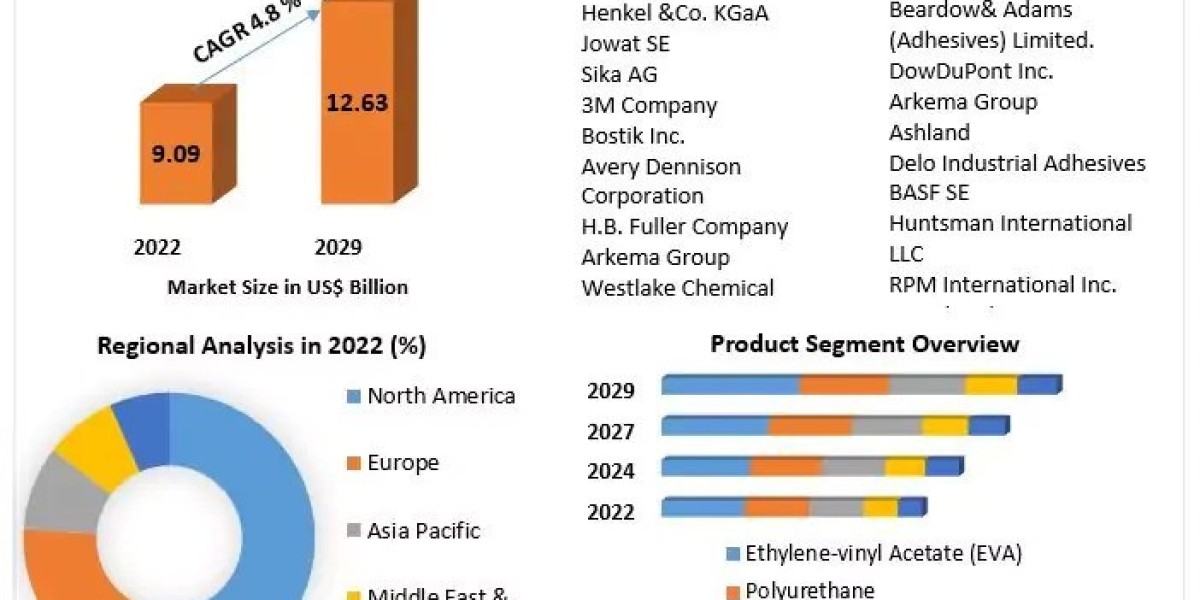
For a better understanding of the market adoption of real world evidence solutions market, the market is analyzed based on its worldwide presence in the countries such as North America (United States, Canada, and Rest of North America), Europe (Germany, France, Italy, Spain, United Kingdom, and Rest of Europe), Asia-Pacific (China, Japan, India, Australia, and Rest of APAC), and Rest of World. North America constitutes a major market for the real world evidence solutions industry owing to the presence of key players in the United States. The growing number of RWE service providers in the region, as well as favorable government regulations, are expected to contribute to market growth. The transition from volume-based to value-based care is expected to drive market growth. Furthermore, the growing geriatric population and the resulting rise in the prevalence of chronic diseases are driving the growth of this market.
Some of the major players operating in the market include IQVIA, IBM, PPD Inc., Parexel Internation Corporation, PerkinElmer Inc., Icon Plc, Syneos Health Inc, Cegedim Health Data, Medpace Holdings Inc, Oracle Corporation.
Access a Sample copy of the report browse through- https://univdatos.com/get-a-free-sample-form-php/?product_id=30787
Global Real World Evidence Solutions Market Segmentation
Market Insight, by Component
- Services
- Data Sets
Market Insight, by Application
- Pharmaceutical & Medical Device Companies
- Healthcare Payers
- Healthcare Providers
- Others
Market Insight, by Region
- North AmericaUnited States
- Canada
- Rest of North America
- EuropeFrance
- Germany
- Italy
- Spain
- United Kingdom
- Rest of Europe
- Asia-PacificChina
- India
- Australia
- Japan
- Rest of Asia-Pacific
- Rest of World
Top Company Profiles
• IQVIA
• IBM
• PPD Inc.
• Parexel Internation Corporation
• PerkinElmer Inc.
• Icon Plc
• Syneos Health Inc
• Cegedim Health Data
• Medpace Holdings Inc
• Oracle Corporation