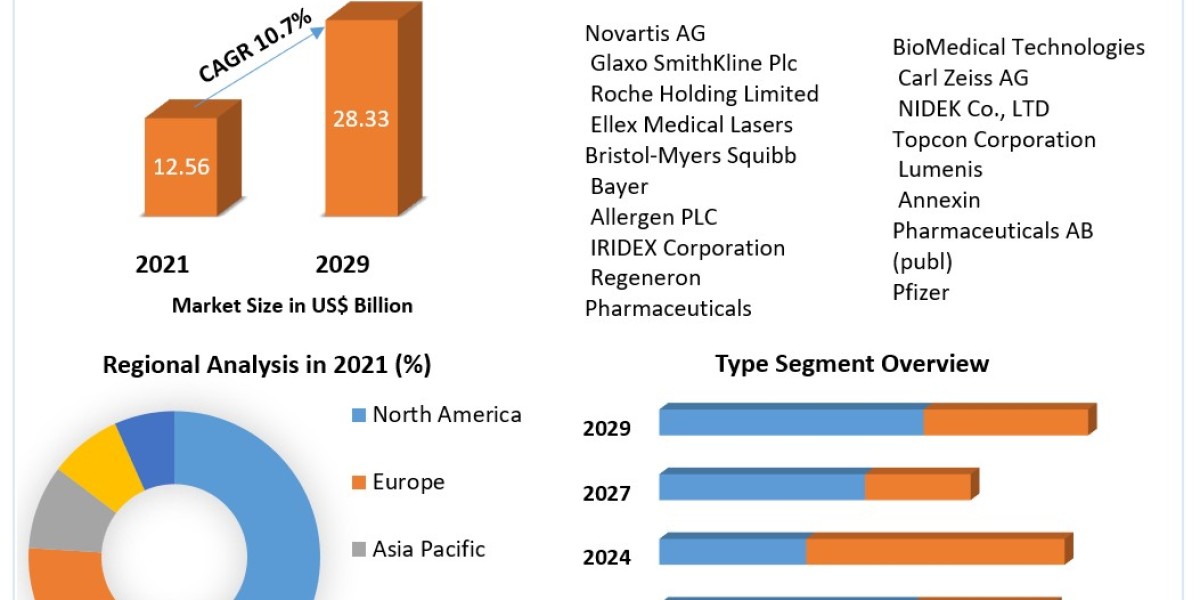
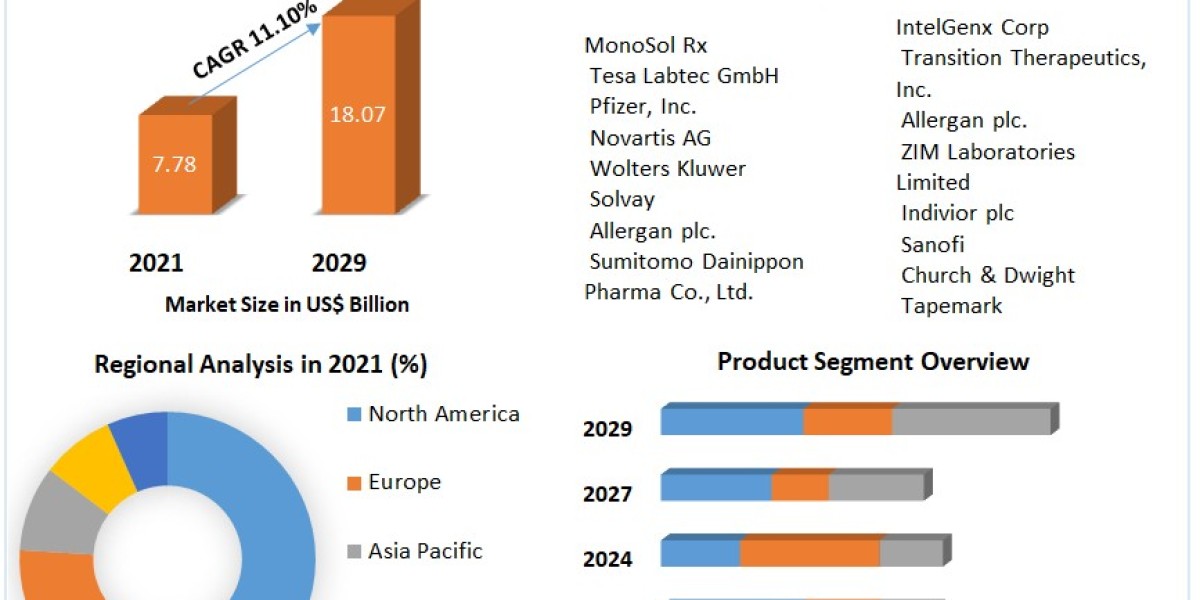
In an increasingly regulated global market, the medical device industry faces significant challenges related to quality management and regulatory compliance. ISO 13485 Certification in Seychelles is the internationally recognized standard for Quality Management Systems (QMS) specific to medical devices. For organizations operating in Seychelles, achieving ISO 13485 certification not only enhances product quality and safety but also demonstrates a commitment to regulatory requirements and customer satisfaction.
ISO 13485 Implementation in Seychelles
Implementing ISO 13485 in Seychelles requires a strategic and well-structured approach tailored to the specific needs of medical device manufacturers and service providers. The journey begins with a thorough gap analysis to assess existing quality management practices against the ISO 13485 standard. This assessment helps organizations identify areas for improvement and formulate a comprehensive implementation plan.
The first step in the implementation process is to establish a quality management policy that reflects the organization’s commitment to quality and regulatory compliance. This policy must be communicated effectively to all employees to foster a culture of quality within the organization.
Next, organizations need to identify and document their processes related to the design, development, production, and post-market activities of medical devices. This documentation serves as a foundation for the QMS and includes procedures, work instructions, and quality manuals. By standardizing processes, organizations can ensure consistency and minimize variability in product quality.
Training is a critical component of ISO 13485 implementation. Employees must be educated about the requirements of the standard, their roles within the QMS, and the importance of adhering to established processes. Creating awareness around quality management helps cultivate a proactive approach to compliance and improvement.
Once the QMS is implemented, organizations in Seychelles should monitor performance through regular reviews and internal audits. These activities not only assess compliance with ISO 13485 Implementation in Turkey but also identify opportunities for continuous improvement. Feedback from stakeholders, including customers and regulatory bodies, should be integrated into the management review process to ensure that the QMS remains effective and relevant.
ISO 13485 Services in Seychelles
Seychelles offers a variety of consultancy services specializing in ISO 13485 certification. These firms provide comprehensive support to organizations seeking to achieve and maintain compliance with the standard.
Consultants typically begin with a preliminary assessment to identify gaps in current practices relative to ISO 13485. This analysis helps organizations prioritize actions and develop a roadmap for successful certification. The expertise of consultants can streamline the implementation process, saving time and resources.
Documentation is a crucial service provided by consultants. They assist organizations in creating the necessary documentation, including quality manuals, procedures, and records, that are compliant with ISO 13485 requirements. This documentation is essential for both effective implementation and demonstrating compliance during audits.
In addition to documentation support, training services are tailored to the specific needs of the organization. Consultants conduct workshops and training sessions to educate employees about ISO 13485 Services in Pune, covering topics such as risk management, quality control, and regulatory compliance. By equipping staff with the necessary knowledge and skills, organizations can foster a culture of quality and continuous improvement.
Consultancy firms also provide ongoing support to ensure that the QMS remains compliant with evolving regulations and standards. This support can include regular audits, updates to documentation, and assistance with corrective actions when non-conformities are identified.
ISO 13485 Audit in Seychelles
The audit process is a fundamental aspect of ISO 13485 certification in Seychelles. Organizations must undergo both internal and external audits to validate the effectiveness of their Quality Management System.
Internal audits are conducted by trained personnel within the organization. These audits assess compliance with ISO 13485 requirements, evaluate the effectiveness of processes, and identify areas for improvement. Internal audits provide valuable insights and help organizations ensure that their QMS is functioning as intended.
External audits are performed by accredited certification bodies. During these audits, auditors review documentation, conduct interviews, and observe operations to assess compliance with ISO 13485 standards. They evaluate the organization’s ability to manage quality risks effectively and verify adherence to established processes.
Achieving ISO 13485 certification signifies an organization’s commitment to producing safe and effective medical devices. It enhances the organization’s credibility in the market, fosters trust among customers and regulatory bodies, and opens doors to new business opportunities both locally and internationally.
Conclusion
In conclusion, ISO 13485 Registration in Seychelles is crucial for organizations operating in the medical device sector. Through a systematic approach to implementation, access to specialized services, and a robust audit process, businesses can achieve compliance and enhance their quality management practices. As the demand for high-quality medical devices continues to grow, adopting ISO 13485 is essential for organizations aiming to improve product safety, meet regulatory requirements, and ultimately ensure customer satisfaction. By embracing this standard, organizations in Seychelles can solidify their position as leaders in the medical device industry while contributing to better healthcare outcomes.