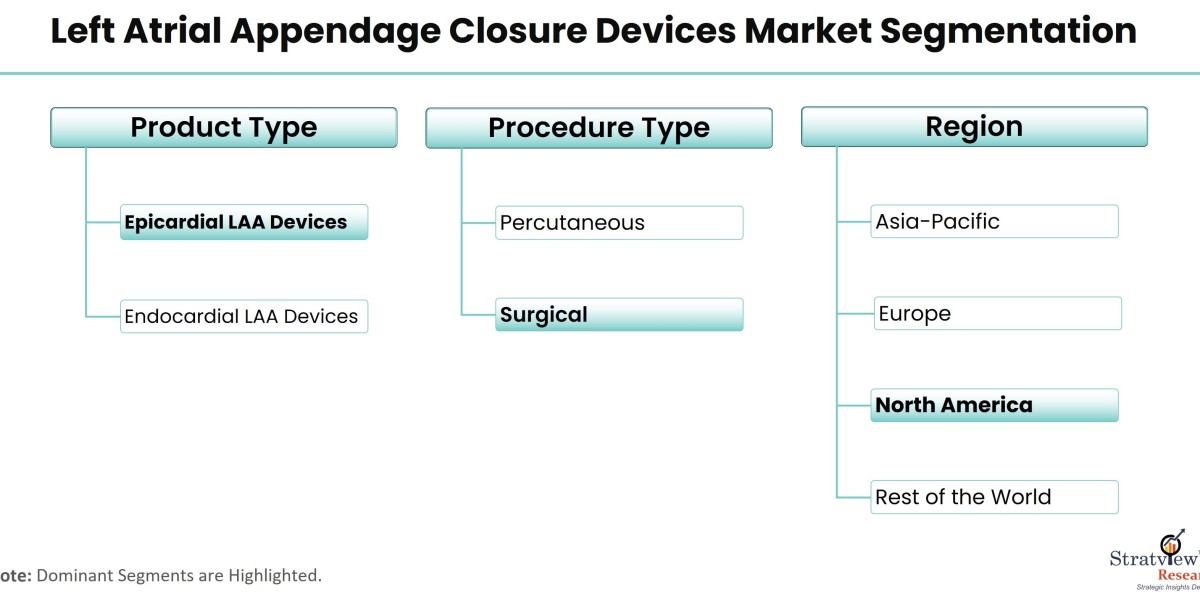
The global left atrial appendage closure devices market is witnessing significant growth as demand for advanced stroke prevention options rises, particularly for patients with atrial fibrillation (AF). Left atrial appendage closure devices are used to close the left atrial appendage, where blood clots can form in AF patients, thereby reducing the risk of stroke. As the market expands in 2024, both opportunities and challenges will play a key role in shaping its future.
According to Stratview Research, the left atrial appendage closure devices market was estimated at USD 1.25 billion in 2022 and is likely to grow at a CAGR of 20.27% during 2023-2028 to reach USD 3.8 billion in 2028.
Opportunities Driving Market Growth
- Increasing Prevalence of Atrial Fibrillation: The growing global incidence of atrial fibrillation, particularly among the aging population, is one of the primary drivers of market growth. AF increases the risk of stroke, and as the elderly population expands, the demand for preventive solutions like left atrial appendage closure devices is surging. In 2024, this trend is expected to intensify, further boosting market expansion.
- Advancements in Device Technology: Technological innovations are paving the way for more efficient, safer, and minimally invasive left atrial appendage closure devices. Newer devices are smaller, easier to implant, and feature advanced materials that reduce complications. These innovations are making left atrial appendage closure procedures more attractive to both healthcare providers and patients, driving adoption rates worldwide. The integration of real-time imaging and better delivery systems is further enhancing the precision and success of these procedures.
- Expanding Reimbursement and Regulatory Support: In recent years, several countries have expanded insurance coverage for left atrial appendage closure procedures, recognizing their cost-effectiveness and long-term health benefits. As reimbursement policies become more favorable in 2024, access to left atrial appendage closure devices is expected to broaden, making them available to a larger pool of patients. Additionally, regulatory bodies like the FDA are providing quicker approvals for new devices, facilitating faster market entry.
- Growing Clinical Adoption: Increasing awareness among cardiologists and electrophysiologists about the benefits of left atrial appendage closure devices for stroke prevention is contributing to their widespread adoption. Clinical evidence supporting their long-term safety and efficacy is encouraging more hospitals to incorporate left atrial appendage closure devices into their treatment protocols.
Challenges Facing the Market
- High Cost of Treatment: Despite the growing adoption of left atrial appendage closure devices, their high cost remains a challenge, especially in emerging markets with budget constraints. While reimbursement policies are improving, the overall expense of the procedure and device may limit accessibility for some patients.
- Regulatory and Procedural Barriers: Although regulatory approval is increasing, there are still barriers to the swift approval of newer devices in certain markets. The complexity of the implantation procedure and the need for specialized training for medical professionals also present challenges that could hinder broader adoption.
Conclusion
The global left atrial appendage closure devices market presents a promising future, driven by technological innovations, rising AF prevalence, and expanding reimbursement policies. However, challenges such as high treatment costs and regulatory hurdles remain. As the market matures in 2024, overcoming these challenges will be crucial in ensuring that left atrial appendage closure devices become a standard solution for stroke prevention in AF patients worldwide.